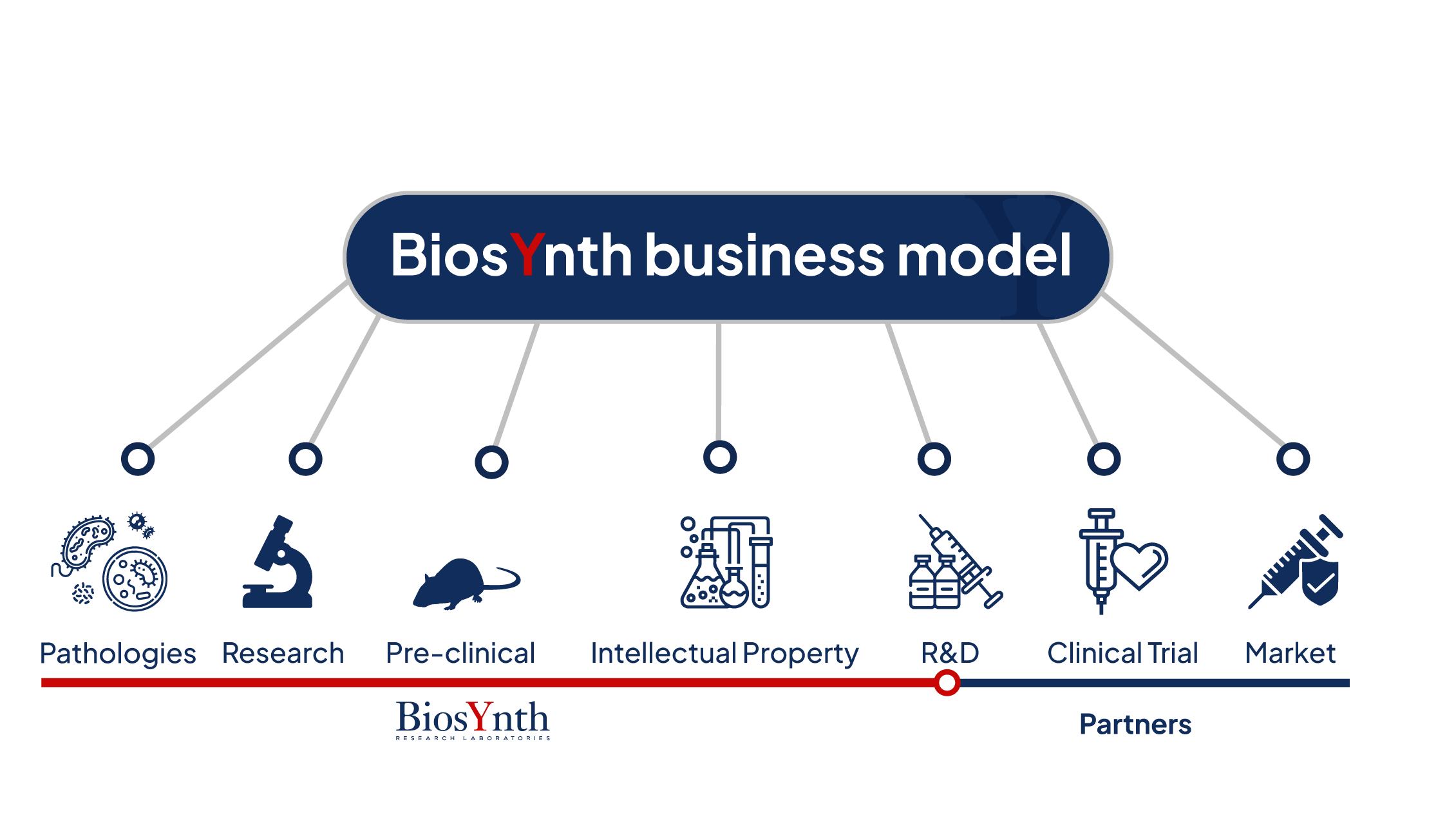
In the context of human vaccine development, BiosYnth's business model is structured across sequential phases, from
scientific discovery to post-preclinical Research and Development. All activities are carried out in facilities that operate
in compliance with Good Laboratory Practice (GLP) standards, ensuring the quality, traceability, and reliability of the
data generated throughout the development process.
Here's how the process can be outlined:
1. Pathology : The first phase involves identifying a specific infectious disease that requires a vaccine based on epidemiological reports released by the WHO. This could be a new infectious disease or a known disease for which there is no effective vaccine yet, an emerging disease, or a disease for which existing vaccines could be improved. The selection of the disease thus guides the direction of basic research and subsequent development.
2. Research : The research phase focuses on understanding the pathogen responsible for the disease, such as viruses or bacteria, and how the human immune system responds to it. This includes the identification of phenotype-specific antigens that can be used to provoke a protective immune response in the host organism (body).
3. Pre-clinical : During the pre-clinical phase, vaccine candidates are tested in vitro and in animal models to assess their safety and ability to induce a specific and potentially effective immune response. These studies are crucial to determine which formulations to advance to the next phase of clinical trials.
4. Intellectual Property : Protecting discoveries through patents is essential for the developed know-how including antigens, adjuvants, vaccine production methods, and formulations resulting from pre-clinical indications. This protection allows BiosYnth to maintain a competitive edge and attract investments from industrial and commercial partners.
5. R&D (Research and Development) : R&D continues through all stages of pilot-scale vaccine development, including formulation optimization and refinement of production methods. All activities are conducted in GLP-compliant facilities, ensuring scientific rigor, data traceability, and high-quality experimental outcomes.
6. Clinical Trial (with Partners): Given the complexity and high cost of clinical studies, finding strategic partners is crucial. These can be large pharmaceutical companies, national governments, or public and private institutions. These partners can provide funding, research infrastructure, and organized access to clinical study populations.
7. Market (with Partners): After successfully completing clinical studies and obtaining regulatory approval from the relevant authorities (Marketing Authorization), the next step is the commercialization of the vaccine. In collaboration with partners, existing distribution channels structured from previous experiences in the vaccine market are used, along with appropriate marketing strategies to reach a wide audience that can benefit. This phase also includes activities such as large-scale production, cold chain management (for vaccines requiring refrigeration and controlled temperature), and post-marketing surveillance programs to evaluate the long-term effectiveness and safety of the vaccine.

